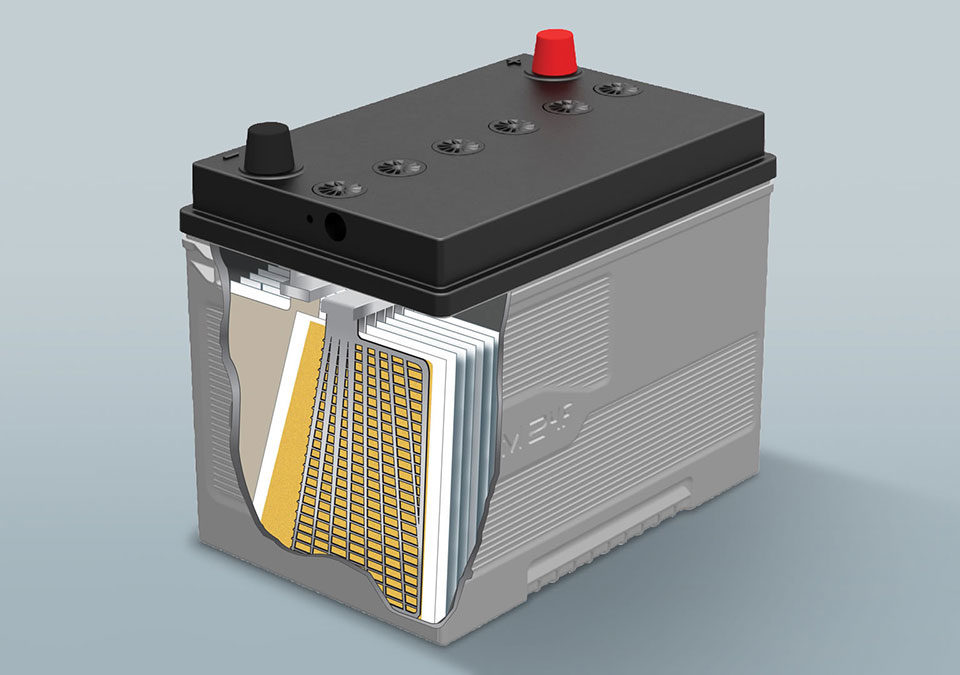
Why does an lead acid battery need water ?

Lead-acid batteries consist of flat lead plates immersed in an array of electrolytes. The electrolyte consists of sulfuric acid and water. Water is an essential part of how a lead battery works. The size of the battery plates and the amount of electrolyte determine the amount of charge lead-acid batteries can store or the number of hours of use.
During the recharging process where electricity flows through the water portion of the electrolyte, the water is converted into its two original elements, oxygen and hydrogen. These gases are highly flammable and are the reason marine or recreational vehicle batteries should be ventilated outside. The use of gases leads to water loss, which is why lead-acid batteries need to be added to water periodically. Low maintenance batteries such as AGM batteries are an exception as they have the ability to compensate for water loss.